anticancer and molecular docking studies on the rare, endangered plant Thymus decussatus Abo-Alez I.N., El-Baz, A.F., Dalia D. Mohamed, Doaa D. Mohamed, Abd El Maksoud, Ahmed I.

E G Y P T I A N J O U R N A L O F B O T A N Y ( E J B O ) Micropropagation, phytochemical, in vitro anticancer and molecular docking studies on the rare, endangered plant Thymus decussatus Abo-Alez I.N., El-Baz, A.F., Dalia D. Mohamed, Doaa D. Mohamed, Abd El Maksoud, Ahmed I. Industrial Biotechnology Departement, Genetic Engineering and Biotechnloogy Research Institute, University of Sadat City, Egypt The axenic culture of Thymus decussatus, a rare wild species found only above 1500 m altitude in the St. Katherine Protectorate, Egypt, was established. Full MS (Murashige and Skoog medium) medium supplemented with 0.35 mg/l benzyladenine (BA) and 0.2 mg/l kinetin (Kin) was found to be optimal for micropropagation and acclimatization. Hexane extract showed the most significant cytotoxic effects against hepatocellular carcinoma HepG2 cells with an IC50 of 12 ± 0.69 μg/ml and selectivity over normal hepatocytes (THLE2 IC50=38.57 ± 1.65 μg/ml). High-performance liquid chromatography identified the main components as flavonoids and phenolic acids, with rosmarinic acid being the most abundant (23.7 μg/g dry weight). The combination of hexane extract with sorafenib had a synergistic effect, decreasing the IC50 from 9.289 ± 0.51 to 2.274 ± 0.13 μg/ml. In contrast, an antagonistic effect was seen with doxorubicin, increasing its IC50 from 0.716 ± 0.04 μg/ml to 6.499 ± 0.36 μg/ml. The hexane extract exhibited S-phase cell cycle arrest and apoptosis induction. Analysis of gene expression profiles showed upregulation of p53 and MDR1 genes. Molecular docking analysis revealed that the flavonoids quercetin, chlorogenic acid, apigenin, and rosmarinic acid have high-affinity binding to pro- and anti-apoptotic proteins, suggesting possible mechanisms of action. In conclusion, this work supports conserving this rare species and investigating its phytochemicals as potential adjuvants to improve hepatocellular carcinoma treatment. Keywords: Thymus decussatus; Axenic culture; Micro-propagation; Sorafenib; Rosmarinic acid INTRODUCTION Thymus decussatus is a delicate shrub endemic to high mountain regions of the St Katherine Protectorate in Egypt and an adjacent area of Saudi Arabia, found only above 1500m altitude (1). Due to the extreme aridity, rainfall is very sporadic (every 1015 years), which makes seedling recruitment challenging. A survey identified ten surviving patches, several newly discovered in 2011 after an 8-year drought caused high mortality rates (1). As such, T. decussatus can be considered a critically endangered species in this region. Various Thymus species are used in traditional medicine to treat conditions including abdominal pain, bronchitis, kidney ailments and urinary antisepsis (2,3). Phytochemical studies on T. decussatus identified methoxyflavone 3, 4'dimethoxychrysin as a major compound in methylene chloride fraction of Thymus decussatus methanol extract and other flavonoids and phenolic acids (4). Screening using the sulforhodamine B colorimetric assay revealed potent in vitro cytotoxicity against cancer cell lines, including Ehrlich ascites, brain tumor U251 and hepatocellular carcinoma HepG2 cells (4). Other studies with the related species T. capitatus and T. daenensis found moderate to solid anticancer effects against HepG2 cells and apoptosis induction (5,6). Micropropagation refers to rapidly multiplying plant materials to produce many progeny plants using plant ARTICLE HISTORY Submitted: September 07, 2023 Accepted: March 12, 2024 CORRESPONDANCE TO Islam N. Abo-Alez, Industrial Biotechnology Departement, Genetic Engineering and Biotechnloogy Research Institute, University of Sadat City, Egypt Email: islamnasef300@gmail.com DOI: 10.21608/ejbo.2024.234993.2478 EDITOR Prof. AlBaraa ElSaied, Department of Botany and Microbiology, Faculty of Science (Boys), Al-Azhar University, Cairo, Egypt Email: baraa_elsaied@yahoo.com ©2024 Egyptian Botanical Society tissue culture (7). Phytochemicals are chemical compounds produced by plants, many of which have been shown to have therapeutic effects (4). Cytotoxicity measures how toxic a substance is to living cells and can be tested in cancer cell lines to assess potential anticancer effects. Under controlled aseptic conditions, Plant tissue culture systems now allow for raising plants, organs, tissues, or cells. Additionally, plant development can be controlled by adding plant growth regulators—natural or artificial phytohormones—at specific growth or maturity stages. In the last two decades, there have been many publications on micropropagation of diverse species of the thyme genus: T. bleicherianus (8), T. caespititius (5), T. daenensis (6), and T. hyemalis (9). The present study has been designed to develop a suitable in vitro technique for large-scale multiplication of the rare medicinally active thymus decussatus. Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer deaths worldwide (10). However, treatment options are minimal. The multi-kinase inhibitor sorafenib is the first-line therapy, but efficacy is under 30% due to acquired resistance, mediated in part by ABC exporter pumps (11). Also, Doxorubicin's chemoresistance and cardiotoxicity side effects limit its widespread application as a chemotherapeutic agent (12). As such, substantial investigation has been made into synergistic The Egyptian Journal of Botany (EJBO) is published by the Egyptian Botanical Society. Egypt. J. Bot., Vol. 64, No.3, pp. 1-11 (2024)

combinations to improve sorafenib's efficacy. Natural products like those found in T. decussatus may serve as safer adjuvant options. In silico, molecular docking has become an increasingly important tool for drug discovery. The molecular docking approach is used to model the interaction between a small molecule and a protein at the atomic level, which allows us to characterize the behavior of small molecules at the binding site of target proteins and elucidate fundamental biochemical processes (13). While tissue culture techniques have been described for various thyme species (7), no prior published reports exist for the endangered T. decussatus. Therefore, the present study had two main objectives: first, to develop an optimal tissue culture protocol for micropropagation and acclimatization of T. decussatus to support conservation efforts, and second, to explore further the anticancer effects of T. decussatus extracts, especially in combination with conventional HCC drugs like sorafenib and doxorubicin to elucidate potential synergistic actions. Using an in-silico molecular docking method, the interaction between multiple bioactive substances found in Thymus decussatus and multiple target proteins was also assessed. MATERIALS AND METHODS Micropropagation Sample collection: T. decussatus samples were collected during the flowering stage in April 2018 within the St. Katherine Protectorate Mountain region of Egypt. The plant was identified by officials of St. Katherine Protectorate. Explant sterilization: Shoot apices and nodal explants were cut and immersed in a solution containing activated charcoal (0.1%) (an adsorbent of inhibitory compounds in the culture medium) and citric acid (0.1%) (antioxidant) for 1 hour. After repeated rinsing (five times) with distilled water, explants were sterilized with different NaOCl concentrations (0.75% and 1.5%) for different periods (15, 20 and 25 minutes) followed by 0.1% HgCl2 for different periods (30, 60 and 90 seconds) to optimize a protocol for sterilization (14). Culture media and conditions: Explants were cultured on Murashige and Skoog basal media (MS media) under 16-hour photoperiods (15). Different concentrations and combinations of plant growth regulators, including benzyladenine (BA), naphthalene acetic acid (NAA), kinetin (Kin) and indole butyric acid (IBA), were tested to optimize shoot proliferation, length and leaf production. 2 Rooting was examined on hormone-free media or media supplemented with 0.5 mg/l NAA or IBA. After fungicide treatment, rooted shoots were transferred to a 1:1 vermiculite soil potting mix for hardening and acclimatization. Cytotoxicity assays Chemicals: High glucose Dulbecco’s modified Eagle’s medium (DMEM) was obtained from (Invitrogen/Life Technologies) and fetal bovine serum (FBS) from (Hyclone). Penicillin/streptomycin (Pen/Strep) and trypsin were ordered from Lonza (Basel, Switzerland). Sorafenib 200 mg pills with the trade name Nexavar were obtained from Bayer Schering Pharma (Germany). Doxyrubicin 10 mg vial was obtained from Novartis Pharma Sandos Division. All of the other chemicals and reagents were from Sigma or Invitrogen. Stock solutions of sorafenib and doxorubicin in DMSO were prepared and diluted using DMEM to obtain the required working concentrations. Extracts preparation: Thymus decussatus was airdried and ground into a fine powder. The powdered plant was extracted with methanol, chloroform, hexane, ethyl acetate, and acetone (1 gm of powdered plant: 10 ml solvent) using the maceration extraction method for 72 hours. Then, the solvents were evaporated, and the residues were obtained as extracts from the respective solvents. To achieve the required concentrations, stock solutions of each extract were made in dimethylsulfoxide (DMSO) and diluted in growth medium. Cancer cell lines: HepG-2 cell lines were obtained from the American Type Culture Collection. At 37°C, in a humidified atmosphere with 5% CO2, stock cells were cultivated in DMEM supplemented with 10% inactivated Foetal Bovine Serum FBS, penicillin (100 IU/mL), and streptomycin (100 µg/mL) until confluent. Cell dissociating solution (0.02% glucose in PBS, 0.02% EDTA, and 0.2% trypsin) was used to separate the cells. After checking the viability of the cells, cells were centrifuged and transferred into a plate with 96 wells (cell density 1.2 – 1.8 × 103 cell/100µl complete growth medium/well) and incubated for 24 hours at 37 °C, 5% CO2. MTT assay The percentage of cell viability was assessed using an MTT assay as described by Van Meerloo et al. 2011 (16). Cytotoxicity against both hepatocellular carcinoma HCC cell line (HEPG-2) and normal hepatic THLE2 cell line (from the American Type Culture Egypt. J. Bot. Vol. 64, No.3 (2024)

Collection) was studied. Briefly, Hepg-2 cells were treated with 100 ul of different plant extract concentrations (100, 25, 6.25, 1.56 and 0.39 μg/mL) and different combinations of known anti-cancer drugs and the most cytotoxic hexane extract (sorafenib at 100, 25, 6.25, 1.56 and 0.39 uM and hexane extract at 100, 25, 6.25, 1.56 and 0.39 μg/mL) or (doxorubicin at 100, 25, 6.25, 1.56 and 0.39 uM and hexane extract at 100, 25, 6.25, 1.56 and 0.39 μg/mL) then incubated for 24 hours in an incubator. The culture medium was then replaced with 100 μl of MTT solution (0.5 mg/mL); after 4 hours at 37°C, the MTT solution was removed, and the MTT Solubilization Solution [M-8910] was added to each well to dissolve the formed formazan crystals for 15 min. Finally, the absorption of the samples was measured at 450 nm using a ROBONIK P2000 Elisa Reader. Then, we calculated the cell viability percentage using the formula: (OD of treated sample/control OD) × 100. The assay was performed three times, and the median inhibitory concentrations (IC50) were calculated. viral oncogene homolog B1(B-raf). Total mRNA from Hepg-2 cell lines was extracted using an RNeasy RNA Extraction Mini Kit (Qiagen, Hilden, Germany). The purity and concentration of extracted RNA were measured using a nanodrop spectrophotometer (Thermo Fisher Scientific Inc. MA, USA). The purified RNA was synthesised into cDNA using reverse transcriptase (iScriptTM One-Step RT-PCR Kit with SYBR® Green, USA). We quantified P53, MDR1 and Braf gene expression levels on an RT-PCR system using Rotor-Gene SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany) with primer pairs in Table 1. The reaction protocol was as follows: cDNA synthesis for 10 min at 50°C then iScript Reverse transcriptase inactivation for 5 min at 95°C then PCR cycling and detection (30 to 45 cycles) for 10 sec at 95°C- 30 sec at 55°C to 60°C then Melt curve analysis for 1 min at 95°C then 1 min at 55°C then 10 sec at 55°C (80 cycles, increasing each by 0.5°C each cycle). The fold changes in P53, MDR1 and B-raf genes were determined using GAPDH as a housekeeping gene. Evaluation of each sample was done three times to ensure accuracy (18). Cell cycle analysis This assay was done using (BioVision Annexin V-FITC Apoptosis Detection Kit, USA). Briefly, Hepg-2 cells grown in 12-well plates were treated for 24 hours with T. decussatus hexane extract (12±0.69 ug/ml). The dose of hexane extract in this assay corresponds to the IC50 that resulted from the viability assay. After being incubated, the cells were separated using trypsin, rinsed with PBS (phosphatebuffered saline; pH = 7.4), and centrifuged. Cells (1-5 x 105) were resuspended in 500 μl of 1X Binding Buffer (10 mM HEPES, 25 mM CaCl2, 140 mM NaCl, pH 7.4) and then incubated with 5 μ Annexin V-FITC and 5 μL of propidium iodide (50 μg/mL) for 5 min. The cells were then analyzed using a flow cytometer (Ex = 488 nm; Em = 530 nm) and detected by the FITC signal detector (17). High-Performance Liquid Chromatography (HPLC) HPLC assay aims to quantify the polyphenolic and flavonoid content in the most cytotoxic hexane extract. Plant samples were wholly washed, dried, and blended into a fine powder. Using an ultrasonicator, two grams of the powdered sample were extracted with 10 ml of hexane (the most cytotoxic extract against the Hepg-2 cell line). 5 µl of the supernatant was then injected after being collected and filtered. An Agilent 1260 series with an Eclipse C18 column (4.6 mm x 250 mm i.d., 5 m) was used for HPLC analysis. The mobile phase of water (A) and 0.05% trifluoroacetic acid in acetonitrile (B) was programmed with the subsequent linear gradient at 0.9 ml/min flow rate: 12–15 minutes (82% A), 15–16 minutes (82% A), and 16–20 minutes (82% A). The multi-wavelength detector was observed at 280 nm. The column temperature was maintained at 40 °C. Standards (Gallic acid, Chlorogenic acid, Rosmirinic acid, Naringenin, Catechin, Methyl gallate, Apigenin, Ellagic acid, Coffeic acid, Pyro catechol, Ferulic acid, Rutin, Vanillin, Daidzein, quercetin, Cinnamic acid, Hesperetin and Kaempferol) were obtained from Sigma. The phenolic compounds were identified by matching their retention times with those of the standards. For the quantification assay, a calibration curve was drawn using a mixture of standards and the results were provided as μg/g of the powdered sample. Apoptosis assays Apoptotic and necrotic cell populations were quantified after annexin-V and propidium iodide staining using flow cytometry. Gene expression analysis Quantitative PCR analysis was conducted to investigate the relative mRNA expression of specific genes upon treatment with T.decussatus hexane extract, sorafenib and their combination. The targeted genes were Tumor protein P53, multidrug resistance gene 1 (MDR1) and v-raf murine sarcoma Egypt. J. Bot. Vol. 64, No.3 (2024) 3

Docking analysis Ligand preparation: Using the AutoDock 4.0.1 docking program, the phytoconstituents (Quercetin, Chlorogenic, Gallic acid, Apigenin, and Rosmarinic acid) that were found in the most cytotoxic extract from Hepg-2 cell line were subjected to molecular docking analysis for studying the drug molecule interaction with prospective protein targets (19). The structure of Quercetin, Chlorogenic, Gallic acid, Apigenin, Rosmarinic acid, Doxorubicin and Sorafenib was provided from the PubChem database in SDF format. Protein Preparation: A crystal structure of rapidly accelerated fibrosarcoma (B-Raf), multidrug resistance mutation 1 (MDR1), and Cellular tumour antigen (p53) was downloaded from the Protein Data Bank (PDB) using the following IDs: 1UWH, 3G60, and 1TUP respectively. Statistical analysis Experimental results were described as mean ± Standard mean error (SEM). The data were analyzed by one-way ANOVA, with a p-value less than 0.05 considered significantly different. RESULTS AND DISCUSSION Sterilization Surface sterilization was a critical first step, as high NaOCl or HgCl2 concentrations or durations negatively impacted explant viability and leaf color. Optimized 1.5% NaOCl for 15-20 mins and 0.1% HgCl2 for 30 secs resulted in 95-97% sterility with good plant survival (Table 2). Plant death owing to the browning phenomenon represented an obstacle to survival, so activated charcoal 1% as an adsorbing agent and citric acid 0.1% or ascorbic acid 0.1% as an antioxidant were necessary to be used in the medium to extend the culture life. This helped to increase the survival rate of all plants (20). Effect of cytokinins on growth All cytokinin treatments enhanced shoot, leaf and root growth on hormone-free media. While BA stimulated shoot proliferation, excess concentration caused vitrification. In contrast, kinetin improved elongation and hardness to withstand transfers (Figure 1). The optimal combination was determined to be 0.35 mg/l BA, 0.2 mg/l Kin and 0.1 mg/l IBA, resulting in high shoot multiplication, leaf production and rooting (Table 3). The shoot height of some thymus species cultured on media supplemented with cytokinins was reported to decrease with 4 increased cytokinin concentrations (21, 22). These results agree with those obtained by Sáez F et al., who found that BA's thymus piperella shoot proliferation capacity was greater than Kin's (22). Rooting and acclimatization At the rooting stage, no significant difference was observed between cultivation on hormone-free MS media and media supplemented with 0.5 mg/L NAA or 0.5 mg/L IBA. However, during acclimatization, we noticed that plants grown on a hormonal medium were more suitable for acclimatization, so it was preferred to make a mixture of hormones during the shooting phase and then subculturing in a hormonefree medium for rooting (Table 4). Rooted plantlets responded well to the acclimatization scheme applied. One month after the beginning of the acclimatization, 70% of the plantlets looked healthy (Figure 1 f,g). Cytotoxicity results Of the different solvent extracts tested against HepG2 cells, hexane extract showed the greatest potency with an IC50 of 12±0.69 μg/ml, followed by acetone, ethyl acetate, chloroform and methanol extracts (Table 5). Co-treatment experiments revealed that the hexane extract could substantially enhance the cytotoxic potency of sorafenib, reducing its IC50 from 9.289±0.51 down to 2.274 ±0.13 μg/ml, indicative of a synergistic interaction. In contrast, an antagonistic effect was observed with doxorubicin, increasing its IC50 against HepG2. Thus, hexane extract may be a beneficial adjuvant to improve sorafenib therapy (Table 5). A variety of therapeutic herbs have also been shown to increase sorafenib sensitivity, such as Crithmum maritimum and dietary flavonoid (apigenin) that shield Swiss albino mice from sorafenib-induced oxidative, hepato-renal, and genetic damage (23, 24). Esculetin, a coumarin derivative extracted from numerous natural plants, including Artemisia scoparia (redstem wormwood), Citrus limonia (mandarin lime) leaves, and Artemisia capillaries (capillary wormwood), was also mentioned to potentiate the chemotherapeutic effects of sorafenib by modulating the angiogenic VEGF and EGFR/RAS/ERK/NF-κB pathways, promoting apoptosis and inhibiting proliferation (25). Cell cycle analysis results Analysis of cell cycle progression revealed a markedly inhibited viable cell count. Furthermore, the findings on cell cycle distribution demonstrated that treatment with hexane extract led to a much higher Egypt. J. Bot. Vol. 64, No.3 (2024)

Table 1. Primer sequences of the targeted genes (p53, MDR1 and B-raf) and the housekeeping gene GAPDH Gene P53 MDR-1 B-raf GAPDH Primer sequence : F 5’-CCTCAGCATCTTATCCGAGTGG -3 : R 5'-TGGATGGTGGTACAGTCAGAGC -3' F 5’-CCC ATC ATT GCA ATA GCA GG- -3’, R 5'-TGT TCA AAC TTC TGC TCC TGA -3'. F 5’-AACGAGACCGATCCTCATCAGC -3’, R 5'- GGTAGCAGACAAACCTGTGGTTG-3'. F 5’- GTCTCCTCTGACTTCAACAGCG-3’ R 5’- ACCACCCTGTTGCTGTAGCCAA-3’ that treatment (6.11% versus 1.07% in the untreated cells) (Figure 3). According to Yunjie Zhang and colleagues, flow cytometry analysis of the Hepg-2 cells treated with sorafenib revealed a rise in the proportion of cells in the G0/G1 phase and a decrease in the proportion of cells in the S phase after treatment for 24 or 48 hours; however, hexane extract arrested the cell cycle in the S phase and decreased the proportion of cells in the G0/G1 phase (26). Gene expression results a b c d f e g Figure 1. In vitro propagation of Thymus decussatus; a) Explants used for the micropropagation; b) plantlets after 4 weeks on free MS medium; c) micropropagated plants for subculturing; d) Multiple shoots, obtained by culturing on solid MS medium containing 0.5 mg/l BA; e) very low number of shoots, obtained by culturing on solid MS medium containing 0.5 mg/l KIN; f, g) plants after acclimatization in plastic pots. percentage of cells in the S phase (51.91% versus 38.66% in untreated cells) (Figure 2). Additionally, cells treated with hexane extract had higher percentages of pre-G1 cells (27.55% as opposed to 1.69% in control cells) (Figure 2). Later, the Annexin V/PI assay verified that the prominent S peak was related to apoptotic induction. Apoptosis and necrosis analysis results An Annexin V-propidium iodide (PI) apoptosis assay was conducted to confirm apoptosis induction. The assay results demonstrated that hexane extract induced early (13.28 versus 0.37% in the untreated cells) and late (8.16% versus 0.25% in the untreated cells) apoptosis; additionally, it was observed that the proportion of necrotic cells had increased following Egypt. J. Bot. Vol. 64, No.3 (2024) Apoptosis induction by hexane extract was confirmed via testing levels of P53 expression. P53 is a protein responsible for tumour suppression and affects many anti-proliferative processes in the cell, including apoptosis. P53 gene activation is critical for the suppression of tumorigenesis (27). P53 expression levels increased 2.35, 5.5 and 7.2 folds upon treatment with hexane extract, sorafenib and a combination of hexane extract and sorafenib, respectively (Table 6). This indicates that P53 mediates the induced apoptosis of the combination. B-Raf gene was downregulated to record a decrease of 0.37, 0.16 and 0.15 folds upon treatment with hexane extract, sorafenib and a combination of hexane extract and sorafenib, respectively. B-Raf gene is an integral gene in the retrovirus-associated DNA sequence protein (RAS)RAF-extracellular-signal regulated kinase (MEK)extracellular-signal-regulated kinases (ERK) pathway), which, upon activation, enhances cancer cell proliferation, migration and cancer invasion (28). This indicates that the combination of hexane extract and sorafenib has an additive effect on cell proliferation and invasion downregulation. The MDR1 gene is responsible for activating different resistance pathways to sorafenib. The enhanced cellular efflux of cytostatic compounds through transmembrane ATP-binding cassette proteins (MDR proteins) mediates multi-drug resistance (MDR) in HCC. These proteins physiologically regulate the liver's absorption, distribution, and excretion of several pharmaceutical substances and endo- and xenobiotics. On the other hand, an aggressive tumour phenotype and worse overall survival are linked to the overexpression of MDR proteins in liver cancer (29). MDR1 was downregulated to record a decrease of 0.23, 0.38 and 0.21 folds upon treatment with hexane extract, sorafenib and a combination of hexane extract and sorafenib, respectively. 5

Table 2. Influences of different disinfectants (NaOCl – Sodium hypochlorite; HgCl2 – Mercuric chloride) and their exposure time on Thymus decussatus explants Sterilization method Exposure time 0.75% NaOCl 15 minutes 20 minutes 25 minutes 1.5% NaOCl 15 minutes 20 minutes 25 minutes 1.5% NaOCl Then 1 % HgCl2 10 minutes 30 seconds 15 minutes 30 seconds 20 minutes 30 seconds 10 minutes 1 minute 15 minutes 1 minute 20 minutes 1 minute 10 minutes 2 minutes 15 minutes 2 minutes 20 minutes 2 minutes Results Survival % Contaminated % Dead % 79 % survival 21 % contaminated 88 % survival 12 % contaminated 92 % survival 8% contaminated 84 % survival 5 % dead 11 % contaminated 87 % survival 13% dead 94 % survival 6 % dead 96 % survival 4% contaminated 97 % survival 3% contaminated 95 % survival 5% contaminated 86 % survival 14 % dead 88 % survival 12% dead 79 21 74 26 70 % survival 30 % dead 60% survival 40 % dead Table 3. The effect of different concentrations of cytokinins on shoot proliferation, shoot length and number of leaves of thymus decussatus plant Growth regulators Shoot proliferation Shoot length(cm) Number of leaves control BA 0.25 mg/l BA 0.50 mg/l BA 1.00 mg/l Kin 0.25 mg/l Kin 0.50 mg/l BA 0.3 mg/l+ NAA 0.3 mg/l BA 0.35 mg/l + kin 0.2 mg/l + IBA 0.1 mg/l 5.73±1.27 6.80±2.27 8.33±2.66 5.46±1.40 2.53±1.18 1.53±0.51 6.00 ± 1.4 6.8±1.6 4.75±1.09 3.86±1.06 3.00±0.92 2.26±0.77 5.13±1.24 5.33±1.44 3.86±1.66 4.261±1.09 29.133 ±12.6 36.2 ± 8.7 49 ± 11.3 15.6 ± 6.7 20 ± 7.12 14.8 ± 5.1 30.26±6.5 37.7±14.9 HPLC analysis of the flavonoid content in the hexane extract The hexane extract was subjected to HPLC analysis for the quantification of individual polyphenolics like (Chlorogenic acid, Gallic acid, Methyl gallate, Catechin, Coffeic acid, Rutin, Pyro catechol, Ellagic acid, Vanillin, Ferulic acid, Naringenin, Rosmirinic acid, Daidzein, Cinnamic acid, Quercetin, apigenin, hesperetin and Kaempferol). The HPLC results showed that rosmarinic acid (a polyphenolic compound) has the highest concentration at 4.74 6 μg/ml, equivalent to 23.7 µg/g plant powder (Figure 4), followed by Quercetin (a flavonoid compound) at 2.79 μg/ml and Gallic acid at 1.39 μg/ml. The affinity of these polyphenols for B-Raf, MDR1, and P53 was studied by docking analysis. Docking analysis results Molecular docking studies provide helpful insight into interactions between biologically active compounds and protein targets. Analysis of the significant T. decussatus flavonoids revealed strong predicted binding affinity for the pro-apoptotic p53 and anti- Egypt. J. Bot. Vol. 64, No.3 (2024)
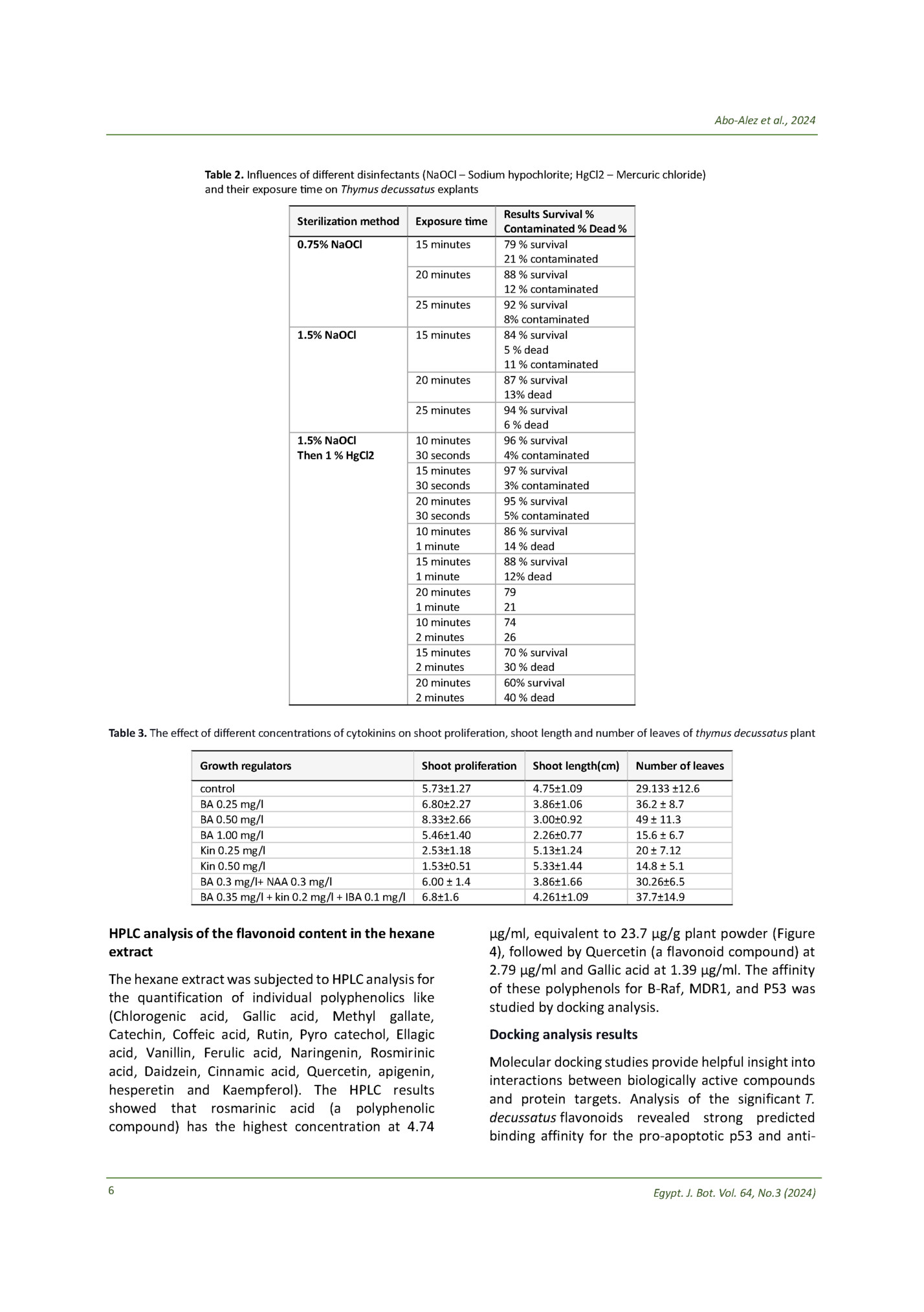
Table 4. The effect of different concentrations of auxins (naphthalene acetic acid (NAA) and indolebutyric acid (IBA)) on the number of roots and root length during the rooting stage of thymus decussatus plant Q1-RR Q1-UR Q1-RR Root length (cm) 5.00 ±0.71 6.18 ± 0.46 6.00 ± 0.26 Q1-LL Q1-LL No. Roots Free MS 0.5 mg/l NAA 0.5 mg/l IBA Q1-UR Q1-UL Basal medium Q1-UL 0.60 ± 0.08 0.50 ± 0.08 0.60 ± 0.07 Table 5. Cytotoxicity of the thymus decussatus hexane extract in combination with sorafenib and doxorubicin drugs on Hepg-2 and THLE2 cell lines Sample Ethyl acetate Acetone Methanol Hexane Chloroform Sorafenib doxorubicin Hexane extract+ Doxorubicin Hexane extract+ Sorafenib Cytotoxicity IC50 HepG2 20.6±1.18 ug/ml 15±0.86 ug/ml 78.9±4.51 ug/ml 12±0.69 ug/ml 52.8±3.02 ug/ml 9.289±0.51 uM 0.716±0.04 uM 6.499±0.36 uM THLE2 38.57±1.65 ug/ml 22.66±0.97 uM 2.274±0.13 uM Hexane extract/HepG2 Control. HepG2 Apoptosis Total Early 27.55 13.28 1.69 0.37 Necrosis Late 8.16 0.25 6.11 1.07 Figure 3. Cytograms showing annexin-V/PI-stained cells HepG-2 cells. Quadrant charts show Q1-UL (necrotic cells- AV-/PI+), Q1-UR (late apoptotic cells- AV+/PI+), Q1-LL (normal cells- AV-/PI-), and Q1-LR (early apoptotic cell- AV+/PI-). Hexane extract Table 6. Relative p53, MDR1 and B-raf gene expression in the HEPG-2 cell line according to sample data Sample Code Hexane Extract Sorafenib Hexane extract+sorafenib Control RT-PCR Fold Change HepG2 p53 MDR1 B-raf 2.3564 0.2392 0.3748 5.5197 0.3884 0.1638 7.2874 0.2189 0.1526 1 1 1 Control Sample data Hexane extract/ HepG2 Control HepG2 DNA content %G0%S G1 36.96 51.19 42.91 38.66 %G2/M 11.85 %PreG1 27.55 18.43 1.69 Comment cell growth arrest at S phase --- Figure 2. Cell cycle analysis of control Hepg-2 cell line and sample Hepg-2 cell line upon treatment with hexane extract. Egypt. J. Bot. Vol. 64, No.3 (2024) apoptotic B-Raf proteins and the drug efflux transporter MDR1 (Table 7). This binding activity likely contributes to the downregulation of these targets as measured in gene expression assays, especially B-Raf. Docking of Compounds into (B-Raf) Binding Site According to (Table 7), the highest docking scores were -9.3 kcal/mole for Quercetin. Quercetin interacts with the backbone of B-Raf via H-bonding 7
Sample min Polyphenolics Gallic acid Chlorogenic acid Catechin Methyl gallate Coffeic acid Pyro catechol Rutin Ellagic acid Vanillin Ferulic acid Naringenin Rosmarinic acid Daidzein Quercetin Cinnamic acid Apigenin Kaempferol Hesperetin Sample Area 20.25 10.10 1.89 6.65 12.96 8.66 0.00 0.00 9.82 8.85 4.09 43.98 1.00 23.14 0.00 13.87 0.00 0.00 Docking of Compounds into (MDR1) Binding Site Conc. (µg/ml) 1.39 1.38 0.44 0.31 1.10 1.29 0.00 0.00 0.40 0.57 0.44 4.74 0.06 2.79 0.00 0.91 0.00 0.00 Conc. (µg/g) 6.97 6.90 2.20 1.54 5.48 6.43 0.00 0.00 2.01 2.84 2.20 23.70 0.32 13.94 0.00 4.54 0.00 0.00 Figure 4. HPLC analysis of thymus decussatus hexane extract Table 7. Binding energies (kcal/mole) obtained after docking Docking Scores kcal/mole Target Ligands and Proteins Quercetin 14 Chlorogenic Gallic acid. 7 Apigenin 4.5 Rosmarinic acid Doxorubicin Sorafenib B-Raf -9.3 -8.2 -6.1 -9.1 -8.7 -8.8 -7.9 MDR1 -9.2 -8.0 -6.3 -8.9 -7.3 -9.1 -7.4 p53 -7.7 -7.7 -5.8 -7.2 -7.5 -9.0 -6.7 with Gln529 and Cys531and via hydrophobic bonds with Val470, Leu513, Lys482, Ile462, Ala480, Trp530 and Phe594 (Table 9). Chlorogenic appeared to significantly participate in the inhibitory activity as it could form hydrophobic interactions with Ala480 and Trp530 and five hydrogen bonds (H-bonds) with Phe594, Asn579, Ser535, Gln529, and Cys531. Gallic acid formed hydrophobic interactions with Ala480 and Trp530 and H-bonds with Cys531. Apigenin formed hydrophobic interactions with Trp530, Phe594, Ala480, and Ile462 and Two hydrogen bonds (H-bonds) with Glu500 and Cys531. Rosmarinic acid 8 formed hydrophobic interactions with Trp530, Val470, Ala480, and Cys531 and Two hydrogen bonds (H-bonds) with Glu500 and Asp593. Doxorubicin binding interacts with the backbone of P53 via Hbonding with Gly533, Cys531 and Ile462. Doxorubicin also makes hydrophobic bonds with Lys482, Leu596, and Ala480. Sorafenib binding interacts with the backbone of P53 via H-bonding with Phe594. Sorafenib also makes a hydrophobic bond with Trp530 and Ala480 (Table 8). According to (Table 7), the highest docking scores were -9.2 kcal/mol for Quercetin. Quercetin interacts with the backbone of MDR1 via H-bonding with Gln721, Tyr303, and Ser725 and via hydrophobic bonds with Phe724, Phe728, and Phe974. Chlorogenic acid appeared to significantly participate in inhibitory activity as it could form hydrophobic interactions with Leu335, Ala338, Val334, and Gly199 and two hydrogen bonds (H-bonds) with Ser218 and Thr195. Gallic acid formed hydrophobic interactions with Ala927 and Ile140 and H-bonds with Arg144, Ala136, and Asn179. Apigenin forms hydrophobic interactions with Phe974 and Phe728. Rosmarinic acid forms hydrophobic interactions with Ser218, Val334, Ala338 and Pro219. Doxorubicin binding interacts with the backbone of MDR1 via H-bonding with Lys230 and Ser345. Doxorubicin also makes hydrophobic bonds with Ile302, Ile227, Leu221, and Ala225. Sorafenib binding interacts with the backbone of MDR1 via H-bonding with Glu252. Sorafenib also makes a hydrophobic bond with Val225 and Arg213 (Table 8). Docking of Compounds into (p53) Binding Site According to (Table 7), the highest docking scores were -9.0 kcal/mol for Doxorubicin. Doxorubicin interacts with the backbone of P53 via H-bonding with Glu198 and Thr140. Doxorubicin also makes a hydrophobic bond with Val172. Quercetin interacts with the backbone of P53 via H-bonding with Gln721, Ser725, and Tyr303. Quercetin also makes hydrophobic bonds with Phe724, Phe974, and Phe728 (Table 8). Chlorogenic acid appeared to participate significantly in the inhibitory activity as it could form hydrophobic interactions with Lue335, Ala338, Val334, and Gly199 and two hydrogen bonds (H-bonds) with Ser218 and Thr195. Gallic acid formed two hydrophobic interactions with Ala927 and Ile140 and four H-bonds with His141, Arg144, Ala136 and Asn179. Apigenin 4.5 formed two hydrophobic interactions with Phe974 and Phe728. Egypt. J. Bot. Vol. 64, No.3 (2024)
Fleepit Digital © 2021