Microphysiological Systems (MPS) More human relevant in vitro models Technology Journal June 2025

Electrophysiological analysis of atrial and ventricular axoCells cardiomyocytes utilizing 3Brain’s CorePlate enabled HD-MEA 3Brain Beating heart-on-chip to model atrial and ventricular cardiac chambers BiomimiX Advancing ALS Modeling: High-Dimensional Insights with CytoTronics’ Pixel CytoTronics Pathway Profiling of axoCellsTM Sensory Neurons Systasy Bioscience Neurotoxicity Evaluation via Nerve MPS and Machine learning Ushio A 3D neurite outgrowth model for studying motor axon biology Mimetas A high content imaging screening assay to identify novel neuroinflammation modulators using human iPSC-derived microglia Sygnature Discovery Multi-electrode array characterization of a human iPSC-derived motor neuron disease model for ALS drug discovery Axion BioSystems SmartHeart: An innovative high throughput assay to generate and assess cardiac micro tissues from hiPSCs answering to the current challenges of cardiac toxicity and efficacy 4DCell AF on Demand : A Human iPSC-Derived Screening Assay Targeting Atrial Fibrillation innoVitro Characterizing sensory neurons as universal bio-digital sensor to explore PNS applications NETRI Poster: Development of iPSC-derived 3D human brain micro-tissues for drug discovery applications Tessara Therapeutics

Technology Journal 2025 A collection of technical papers describing the utilization of iPSC-derived cells from Axol Bioscience on a range of microphysiological systems (MPS) and end-point analysis systems. The journal highlights the greater human relevance and applicability of such models to advanced research and drug discovery. We thank all the contributors and collaborators who have made this journal happen. For more details, contact operations@axolbio.com Leading MPS systems are powered by axoCellsTM At Axol Bioscience, we focus on: Quality manufacturing We manufacture iPSC-derived cell types from our ISO9001:2015 production facility. Global logistics We supply our products worldwide to pharma, biotech, research and technology providers. A dedicated team With a highly skilled, science-rich team, we work collaboratively to realize better human disease models.

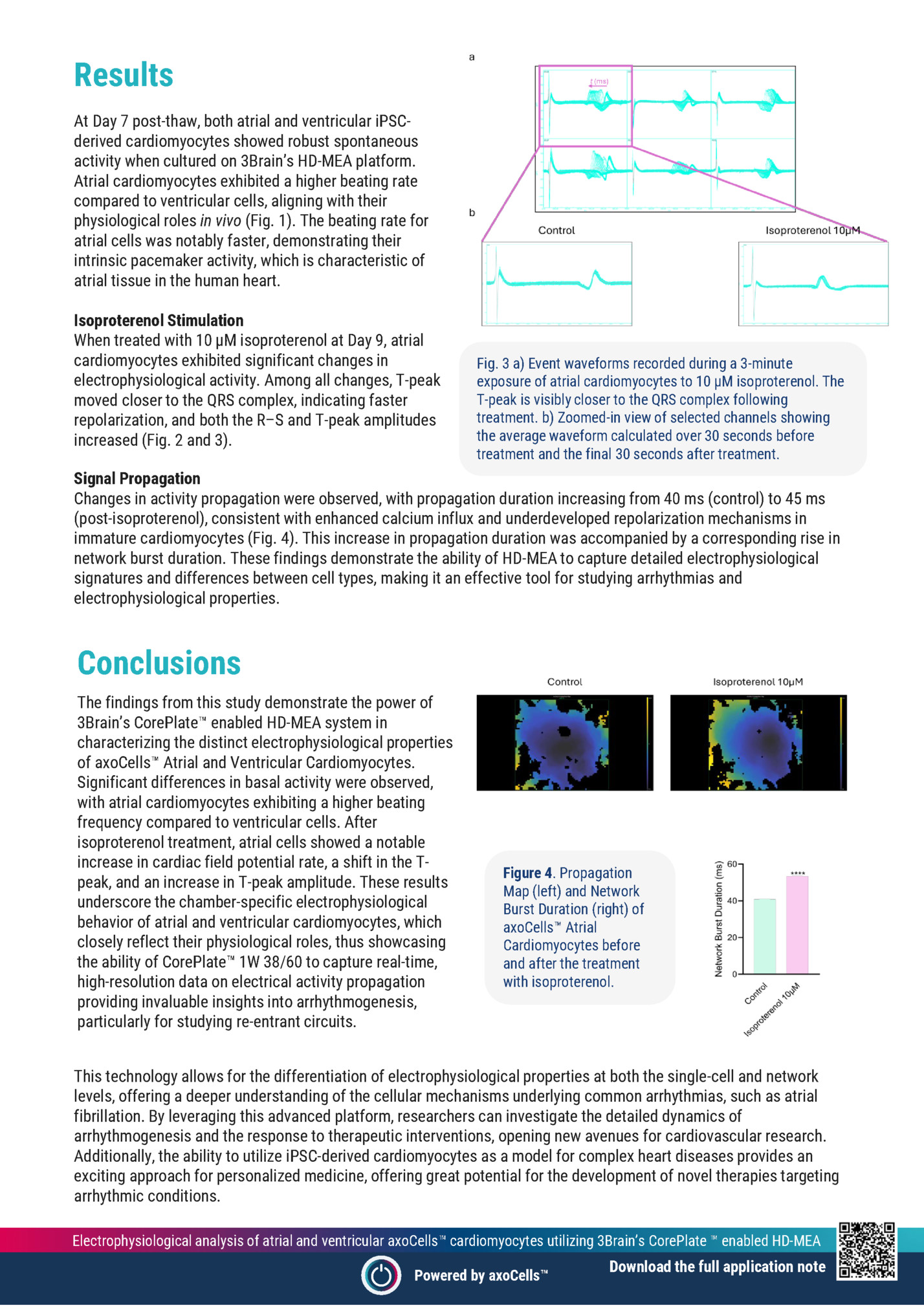
cardiomyocytes utilizing 3Brain’s CorePlate enabled HD-MEA Ivan Verduci, Mariateresa Tedesco 3Brain IT SRL | Genova, Italy Abstract This study evaluates the electrophysiological properties of Axol’s iPSC-derived atrial and ventricular cardiomyocytes using 3Brain’s CorePlate enabled high-density microelectrode array (HD-MEA) technology. Over a 7-day protocol, spontaneous activity and β-adrenergic responsiveness were assessed via (HD-MEA) recordings, and analysis performed with BrainWave5 software. Atrial cells showed a higher spontaneous beating rate than ventricular cells, reflecting chamber-specific functional properties. Both cell types exhibited robust activity, and atrial cardiomyocytes responded predictably to isoproterenol. These findings highlight the effectiveness of combining CorePlate technology and iPSC-derived cardiomyocytes for cardiac modeling and drug screening. Figure 1. a) CorePlate 1W 38/60 b) Activity Map (left) and Raw Signal Map (right) of axoCells Atrial and Ventricular Cardiomyocytes. c) Beating Rate and d) T-Peak amplitude comparison between Atrial and Ventricular Cardiomyocytes. Methods Cell Preparation Atrial (ax2518) and ventricular (ax2508) iPSC-derived cardiomyocytes were thawed and cultured in Axol’s Cardiomyocyte Maintenance Medium (ax2530) on fibronectin-coated 3Brain CorePlate 1W 38/60 arrays. Cells were allowed to mature for seven days post-thaw. Electrophysiological Recording Electrophysiological activity was recorded using the 3Brain BioCAM DupleX system in conjunction with CorePlate 1W 38/60 featuring 4,096 electrodes within a 3.8 × 3.8 mm² active area (21 × 21 µm² electrodes, 60 µm pitch). Recordings were sampled at 20 kHz with a 5 Hz high-pass filter. Figure 2. a) Cardiac Field Potential Rate, b) Inter-Cardiac Field Potential Interval, c) R–T Interval, d) R–S Peak Amplitude, and e) T-Peak Amplitude of axoCells Atrial Cardiomyocytes in the absence and presence of 10 µM isoproterenol. Pharmacological Assay At day 9 in vitro, 10 µM isoproterenol was applied to atrial cardiomyocytes to assess responsiveness to β-adrenergic stimulation. Electrophysiological responses were measured over a 3-minute period before and after treatment. Analysis Software Data were analyzed using BrainWave5 software to extract parameters such as beat frequency, field potential duration, R–T interval, and T-peak amplitude. The same software was also used to extract waveforms and propagation maps. Electrophysiological analysis of atrial and ventricular axoCells cardiomyocytes utilizing 3Brain’s CorePlate enabled HD-MEA Powered by axoCells

At Day 7 post-thaw, both atrial and ventricular iPSCderived cardiomyocytes showed robust spontaneous activity when cultured on 3Brain’s HD-MEA platform. Atrial cardiomyocytes exhibited a higher beating rate compared to ventricular cells, aligning with their physiological roles in vivo (Fig. 1). The beating rate for atrial cells was notably faster, demonstrating their intrinsic pacemaker activity, which is characteristic of atrial tissue in the human heart. Isoproterenol Stimulation When treated with 10 µM isoproterenol at Day 9, atrial cardiomyocytes exhibited significant changes in electrophysiological activity. Among all changes, T-peak moved closer to the QRS complex, indicating faster repolarization, and both the R–S and T-peak amplitudes increased (Fig. 2 and 3). Fig. 3 a) Event waveforms recorded during a 3-minute exposure of atrial cardiomyocytes to 10 µM isoproterenol. The T-peak is visibly closer to the QRS complex following treatment. b) Zoomed-in view of selected channels showing the average waveform calculated over 30 seconds before treatment and the final 30 seconds after treatment. Signal Propagation Changes in activity propagation were observed, with propagation duration increasing from 40 ms (control) to 45 ms (post-isoproterenol), consistent with enhanced calcium influx and underdeveloped repolarization mechanisms in immature cardiomyocytes (Fig. 4). This increase in propagation duration was accompanied by a corresponding rise in network burst duration. These findings demonstrate the ability of HD-MEA to capture detailed electrophysiological signatures and differences between cell types, making it an effective tool for studying arrhythmias and electrophysiological properties. Conclusions The findings from this study demonstrate the power of 3Brain’s CorePlate enabled HD-MEA system in characterizing the distinct electrophysiological properties of axoCells Atrial and Ventricular Cardiomyocytes. Significant differences in basal activity were observed, with atrial cardiomyocytes exhibiting a higher beating frequency compared to ventricular cells. After isoproterenol treatment, atrial cells showed a notable increase in cardiac field potential rate, a shift in the Tpeak, and an increase in T-peak amplitude. These results underscore the chamber-specific electrophysiological behavior of atrial and ventricular cardiomyocytes, which closely reflect their physiological roles, thus showcasing the ability of CorePlate 1W 38/60 to capture real-time, high-resolution data on electrical activity propagation providing invaluable insights into arrhythmogenesis, particularly for studying re-entrant circuits. Figure 4. Propagation Map (left) and Network Burst Duration (right) of axoCells Atrial Cardiomyocytes before and after the treatment with isoproterenol. This technology allows for the differentiation of electrophysiological properties at both the single-cell and network levels, offering a deeper understanding of the cellular mechanisms underlying common arrhythmias, such as atrial fibrillation. By leveraging this advanced platform, researchers can investigate the detailed dynamics of arrhythmogenesis and the response to therapeutic interventions, opening new avenues for cardiovascular research. Additionally, the ability to utilize iPSC-derived cardiomyocytes as a model for complex heart diseases provides an exciting approach for personalized medicine, offering great potential for the development of novel therapies targeting arrhythmic conditions. Electrophysiological analysis of atrial and ventricular axoCells cardiomyocytes utilizing 3Brain’s CorePlate enabled HD-MEA Powered by axoCells Download the full application note
Caterina Pernici1,*, Alberto Mantegazza2,*, Erika Ferrari1, Marco Rasponi1,2, Roberta Visone1, Paola Occhetta1,2 1. BiomimX S.r.l., Viale Decumano 41, 20157, Milano, Italy 2. Department of Electronics, Information and Bioengineering, Politecnico di Milano, Milano, Italy Abstract Developing chamber-specific in vitro cardiac models using hiPSC-derived cardiomyocytes (CMs) remains challenging to accurately predict drug-induced cardiotoxicity. Here, we present atrial and ventricular beating heart-on-chip models (uHearts) developed within the uBeat® Platform, which applies physiological mechanical stimulation to microtissues, to enhance their functionality. The microtissues showed chamber-specific gene expression, synchronous contraction and distinct electrophysiological profiles. uHearts were effectively used to evaluate the cardiotoxic effect of compounds. Figure 1. BiomimX experimental set-up used to generate atrial and ventricular beating heart-on-chip models (uHeart) exploiting iPSC-derived cardiomyocytes from Axol Bioscience. Methods The chamber-specific 3D microtissues were generated using 75% of atrial (or ventricular) human iPSC-CMs (ax2518, ax2508) and 25% of supportive cardiac fibroblasts. Cells were encapsulated in fibrin hydrogel and cultured in the uBeat® Stretch Platforms (BiomimX Srl). A finely tuned mechanical stimulation (i.e. 10% uniaxial strain, 1Hz) was applied for up to 11 days. Microtissues development was monitored daily to assess cell self-organization and to determine the onset of a spontaneous and synchronous beating. PCR analysis was performed to assess ion-channels and chamberspecific structural-related genes (e.g. Figure 2. Experimental plan to develop chamber-specific uHeart models: 1) cells are inserted in the uBeat® stretch platforms, 2) mechanical stimulation is applied up to 11 days; 3) readouts are performed (also upon drug administration). SCN5A, KCNH2, CACNA1C, MYH6, MYH7, MYL2 and MYL7). Immunofluorescence staining was exploited to investigate cell distribution within the microtissue (e.g. Troponin I, typical of cardiomyocytes) and to determine atrial and ventricular phenotypes (i.e. Myosin Light Chain 2 -MLC2-V for ventricle and Sarcolipin-SLN for atrium). Contractile properties were derived by analyzing bright field videos through Muscle Motion software (Sala et al, 2018). Beating period-BP, contraction amplitude-CA, contraction duration-CD and relaxation time-CT were assessed. Electrophysiology was measured by means of a BiomimX proprietary technology, µECG, which allows the recording of microtissue field potentials. BP, spike amplitude-AMP and field potential duration-FPD were evaluated to assess the properties of atrial and ventricular microtissue. Drug-induced alteration of electrophysiology was finally evaluated. Beating heart-on-chip to model atrial and ventricular cardiac chambers Powered by axoCells
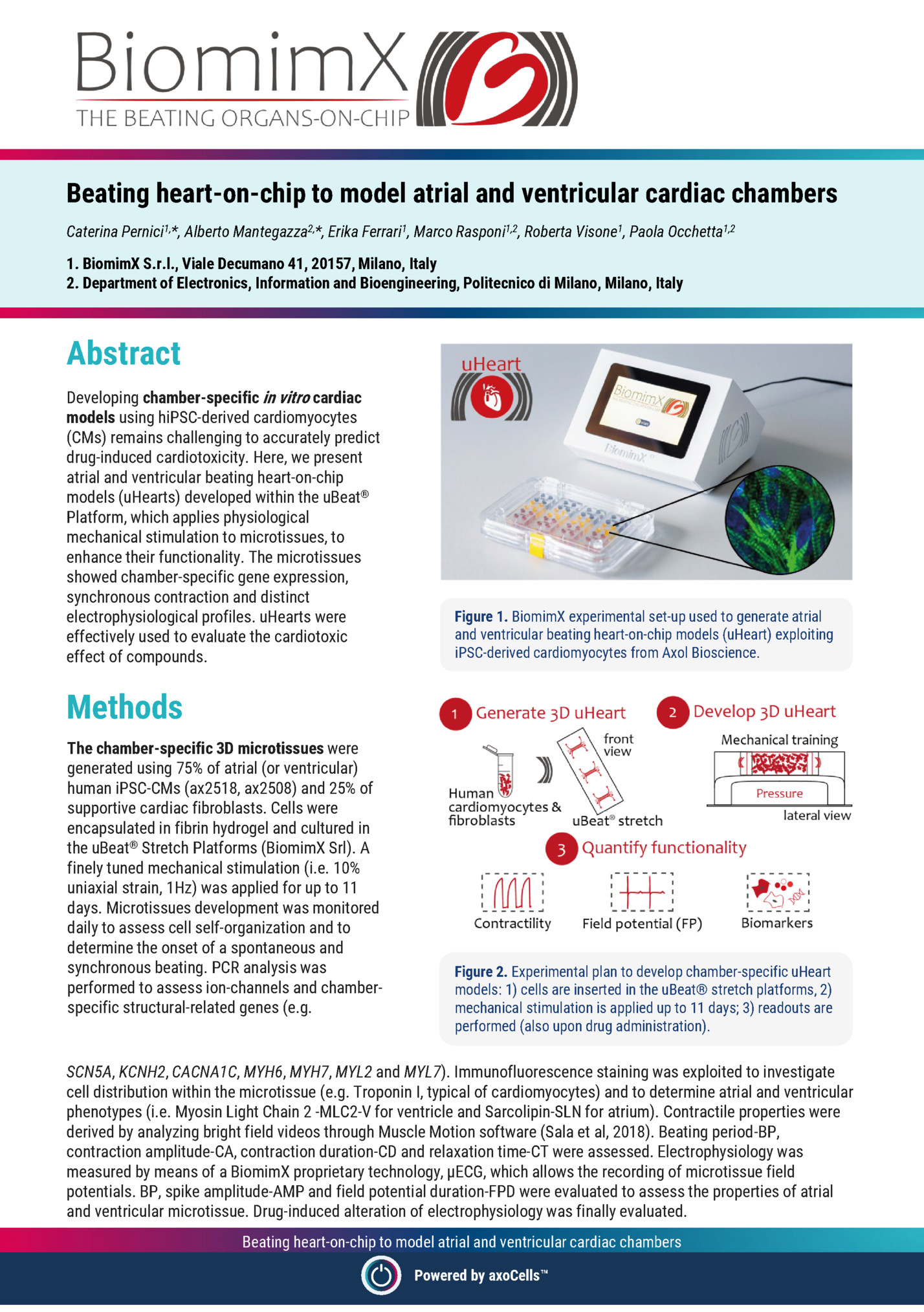
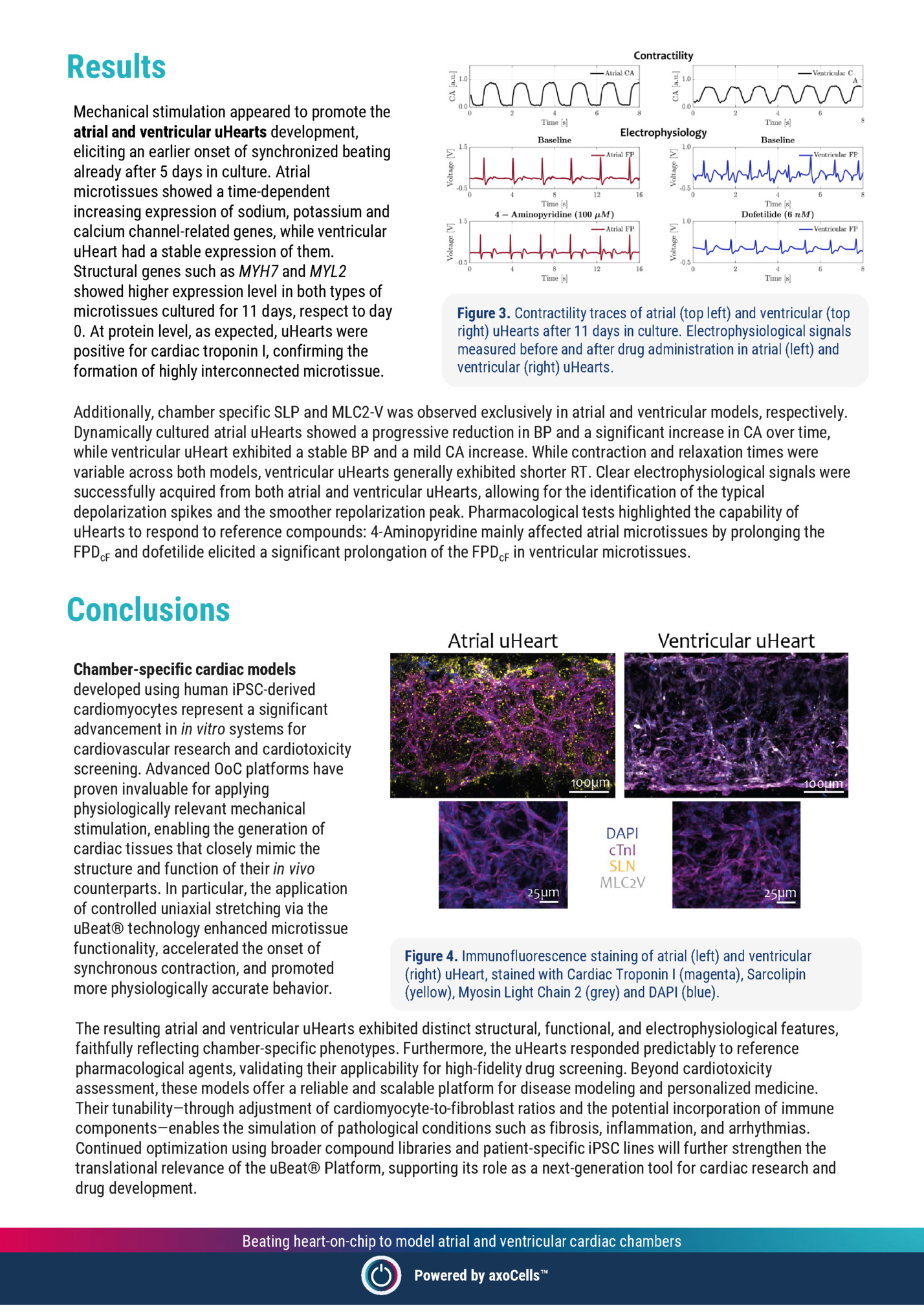
Mechanical stimulation appeared to promote the atrial and ventricular uHearts development, eliciting an earlier onset of synchronized beating already after 5 days in culture. Atrial microtissues showed a time-dependent increasing expression of sodium, potassium and calcium channel-related genes, while ventricular uHeart had a stable expression of them. Structural genes such as MYH7 and MYL2 showed higher expression level in both types of microtissues cultured for 11 days, respect to day 0. At protein level, as expected, uHearts were positive for cardiac troponin I, confirming the formation of highly interconnected microtissue. Figure 3. Contractility traces of atrial (top left) and ventricular (top right) uHearts after 11 days in culture. Electrophysiological signals measured before and after drug administration in atrial (left) and ventricular (right) uHearts. Additionally, chamber specific SLP and MLC2-V was observed exclusively in atrial and ventricular models, respectively. Dynamically cultured atrial uHearts showed a progressive reduction in BP and a significant increase in CA over time, while ventricular uHeart exhibited a stable BP and a mild CA increase. While contraction and relaxation times were variable across both models, ventricular uHearts generally exhibited shorter RT. Clear electrophysiological signals were successfully acquired from both atrial and ventricular uHearts, allowing for the identification of the typical depolarization spikes and the smoother repolarization peak. Pharmacological tests highlighted the capability of uHearts to respond to reference compounds: 4-Aminopyridine mainly affected atrial microtissues by prolonging the FPDcF and dofetilide elicited a significant prolongation of the FPDcF in ventricular microtissues. Conclusions Chamber-specific cardiac models developed using human iPSC-derived cardiomyocytes represent a significant advancement in in vitro systems for cardiovascular research and cardiotoxicity screening. Advanced OoC platforms have proven invaluable for applying physiologically relevant mechanical stimulation, enabling the generation of cardiac tissues that closely mimic the structure and function of their in vivo counterparts. In particular, the application of controlled uniaxial stretching via the uBeat® technology enhanced microtissue functionality, accelerated the onset of synchronous contraction, and promoted more physiologically accurate behavior. Figure 4. Immunofluorescence staining of atrial (left) and ventricular (right) uHeart, stained with Cardiac Troponin I (magenta), Sarcolipin (yellow), Myosin Light Chain 2 (grey) and DAPI (blue). The resulting atrial and ventricular uHearts exhibited distinct structural, functional, and electrophysiological features, faithfully reflecting chamber-specific phenotypes. Furthermore, the uHearts responded predictably to reference pharmacological agents, validating their applicability for high-fidelity drug screening. Beyond cardiotoxicity assessment, these models offer a reliable and scalable platform for disease modeling and personalized medicine. Their tunability—through adjustment of cardiomyocyte-to-fibroblast ratios and the potential incorporation of immune components—enables the simulation of pathological conditions such as fibrosis, inflammation, and arrhythmias. Continued optimization using broader compound libraries and patient-specific iPSC lines will further strengthen the translational relevance of the uBeat® Platform, supporting its role as a next-generation tool for cardiac research and drug development. Beating heart-on-chip to model atrial and ventricular cardiac chambers Powered by axoCells
CytoTronics’ Pixel Christina Banfill, Suma Chinta, Pooja Suresh, Shalaka Chitale CytoTronics | 12 Farnsworth Street Boston, MA 02210 Abstract A Control TDP43 C9ORF72 SOD1 In this application note, we demonstrate the use of CytoTronics’ Pixel to simultaneously capture morphological and electrophysiological data from iPSC-derived motor 100µm neurons from Axol Bioscience. Using impedance-based B electrical imaging and extracellular voltage recordings, we characterized the structural and functional properties of motor neurons from healthy and ALS-associated genotypes (TDP43, C9ORF72, and SOD1) over 28 days. Pixel revealed genotype-specific differences in morphology, network 400µm development, and neuronal firing dynamics, enabling highresolution, longitudinal monitoring of disease-relevant Figure 1. Morphological Phenotypes on DIV20. (A) Brightfield phenotypes. This integrated approach supports applications images of motor neurons on sister glass-bottomed dish on in cell line validation, disease modeling, and phenotypic drug DIV20 showing expected phenotypic clustering patterns. (B) screening. Multi-parametric electrical images of motor neurons showing Methods Human iPSC-derived motor neurons representing healthy control (ax0076), TDP43 (ax0079), C9ORF72 (ax0074), and SOD1 (ax0735) genotypes were plated onto CytoTronics’ Pixel microplates pre-coated with poly-D-lysine and vitronectin. Each well was seeded with either 25,000 or 50,000 cells, corresponding to 190,000 and 380,000 cells/cm2, respectively. Cultures were maintained under standard conditions for 28 days, with medium changes performed according to recommended protocols. To monitor morphological phenotypes and overall cell health, a sister culture was maintained in parallel on a glass-bottomed dish. Pixel and Measurement Overview The Pixel enables simultaneous acquisition of two complementary data types from live cells: impedancebased electrical imaging and extracellular neural measurements. All measurements were performed using the integrated Pixel hardware and software suite. Principal Component 2 distinct phenotypic signatures. Principal Component 1 Principal Component 1 Figure 2. Principal component analysis (PCA) of control and disease motor neurons show distinct clustering and separation of cell lines based on electrical imaging measurements. Electrical Imaging Electrical imaging was conducted every 2 hours throughout the 28-day culture period. This non-invasive, label-free technique captures cell-induced perturbations in electric fields within each well. More than 20 orthogonal impedance measurements were collected per well at each timepoint, providing detailed structural and morphological characterization. Variations in impedance reflect key biological properties such as cell attachment, barrier integrity, morphology, and motility. Different geometries and frequencies of the electric field allow selective probing of these cellular traits. High-dimensional analyses, such as principal component analysis, can then integrate these multiple measurements into a single comprehensive output. Advancing ALS Modeling: High-Dimensional Insights with CytoTronics’ Pixel Powered by axoCells
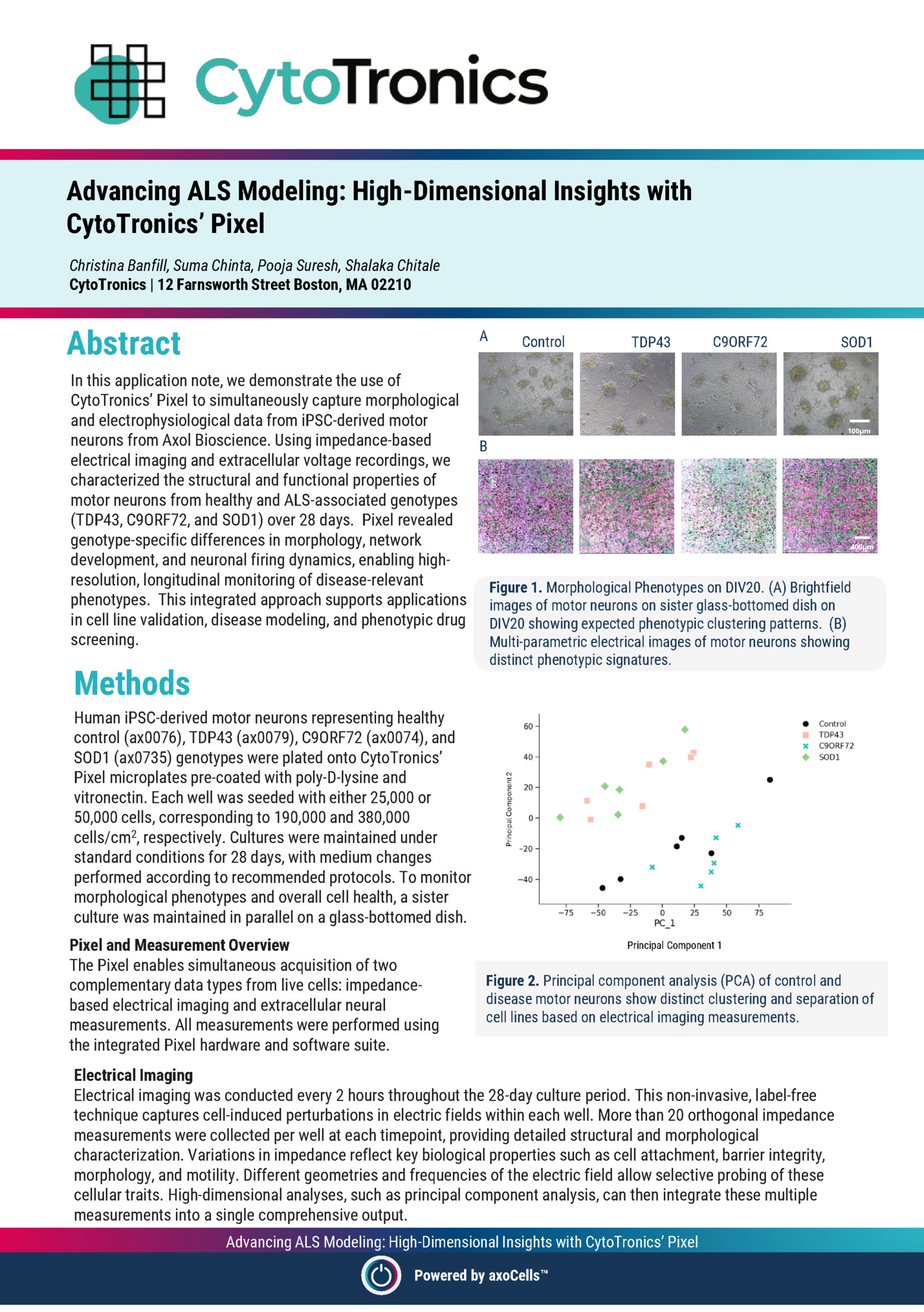
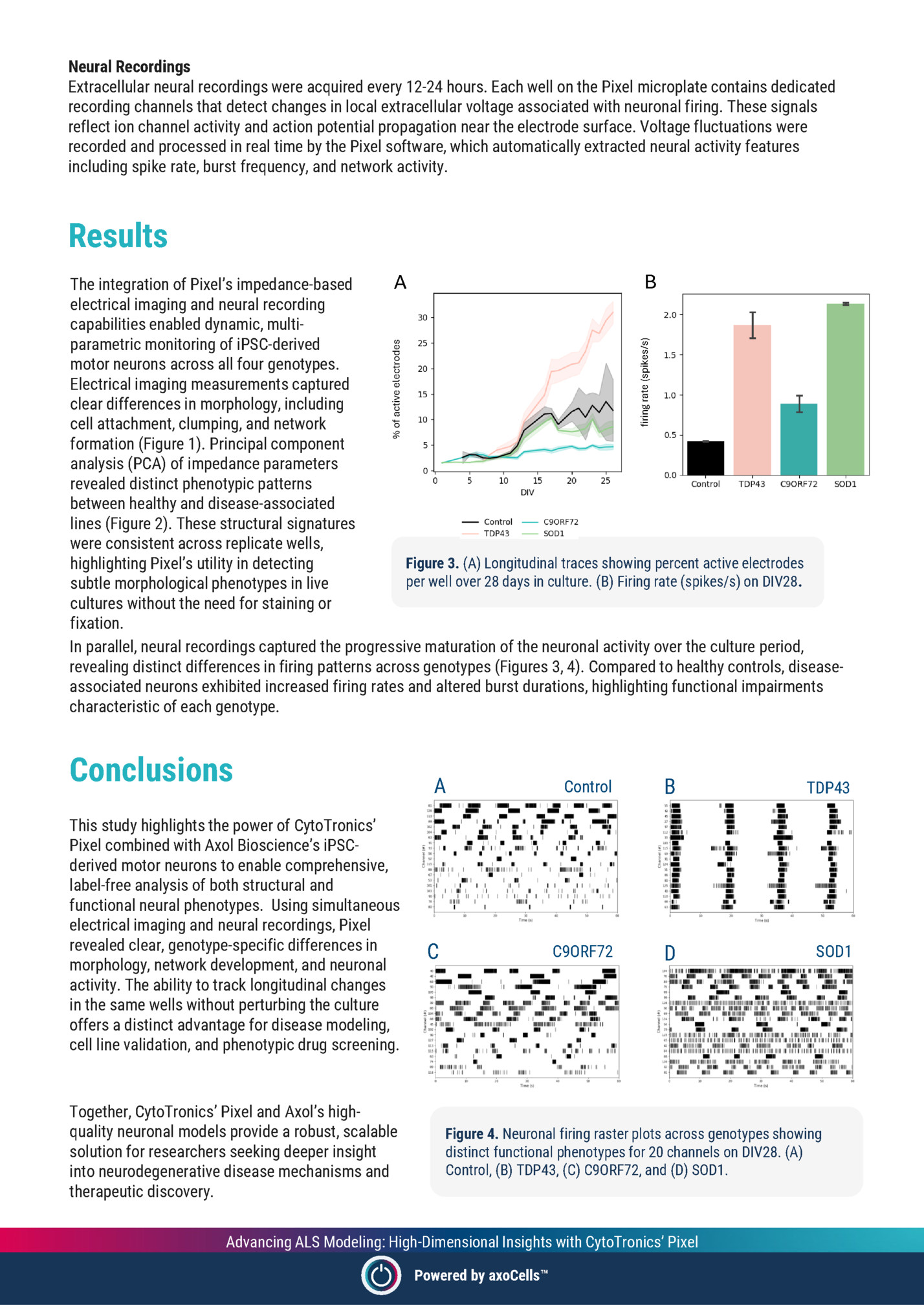
Extracellular neural recordings were acquired every 12-24 hours. Each well on the Pixel microplate contains dedicated recording channels that detect changes in local extracellular voltage associated with neuronal firing. These signals reflect ion channel activity and action potential propagation near the electrode surface. Voltage fluctuations were recorded and processed in real time by the Pixel software, which automatically extracted neural activity features including spike rate, burst frequency, and network activity. Results % of active electrodes firing rate (spikes/s) B A The integration of Pixel’s impedance-based electrical imaging and neural recording capabilities enabled dynamic, multiparametric monitoring of iPSC-derived motor neurons across all four genotypes. Electrical imaging measurements captured clear differences in morphology, including cell attachment, clumping, and network formation (Figure 1). Principal component analysis (PCA) of impedance parameters revealed distinct phenotypic patterns between healthy and disease-associated lines (Figure 2). These structural signatures were consistent across replicate wells, Figure 3. (A) Longitudinal traces showing percent active electrodes highlighting Pixel’s utility in detecting per well over 28 days in culture. (B) Firing rate (spikes/s) on DIV28. subtle morphological phenotypes in live cultures without the need for staining or fixation. In parallel, neural recordings captured the progressive maturation of the neuronal activity over the culture period, revealing distinct differences in firing patterns across genotypes (Figures 3, 4). Compared to healthy controls, diseaseassociated neurons exhibited increased firing rates and altered burst durations, highlighting functional impairments characteristic of each genotype. Conclusions This study highlights the power of CytoTronics’ Pixel combined with Axol Bioscience’s iPSCderived motor neurons to enable comprehensive, label-free analysis of both structural and functional neural phenotypes. Using simultaneous electrical imaging and neural recordings, Pixel revealed clear, genotype-specific differences in morphology, network development, and neuronal activity. The ability to track longitudinal changes in the same wells without perturbing the culture offers a distinct advantage for disease modeling, cell line validation, and phenotypic drug screening. Together, CytoTronics’ Pixel and Axol’s highquality neuronal models provide a robust, scalable solution for researchers seeking deeper insight into neurodegenerative disease mechanisms and therapeutic discovery. A B TDP43 C9ORF72 C Control D SOD1 Figure 4. Neuronal firing raster plots across genotypes showing distinct functional phenotypes for 20 channels on DIV28. (A) Control, (B) TDP43, (C) C9ORF72, and (D) SOD1. Advancing ALS Modeling: High-Dimensional Insights with CytoTronics’ Pixel Powered by axoCells
Fleepit Digital © 2021