flip
flip
Alphabetical Test Index — A Comprehensive Overview of Laboratory Assays, Turnaround Times, Pricing, and Handling Guidelines
Overview and scope
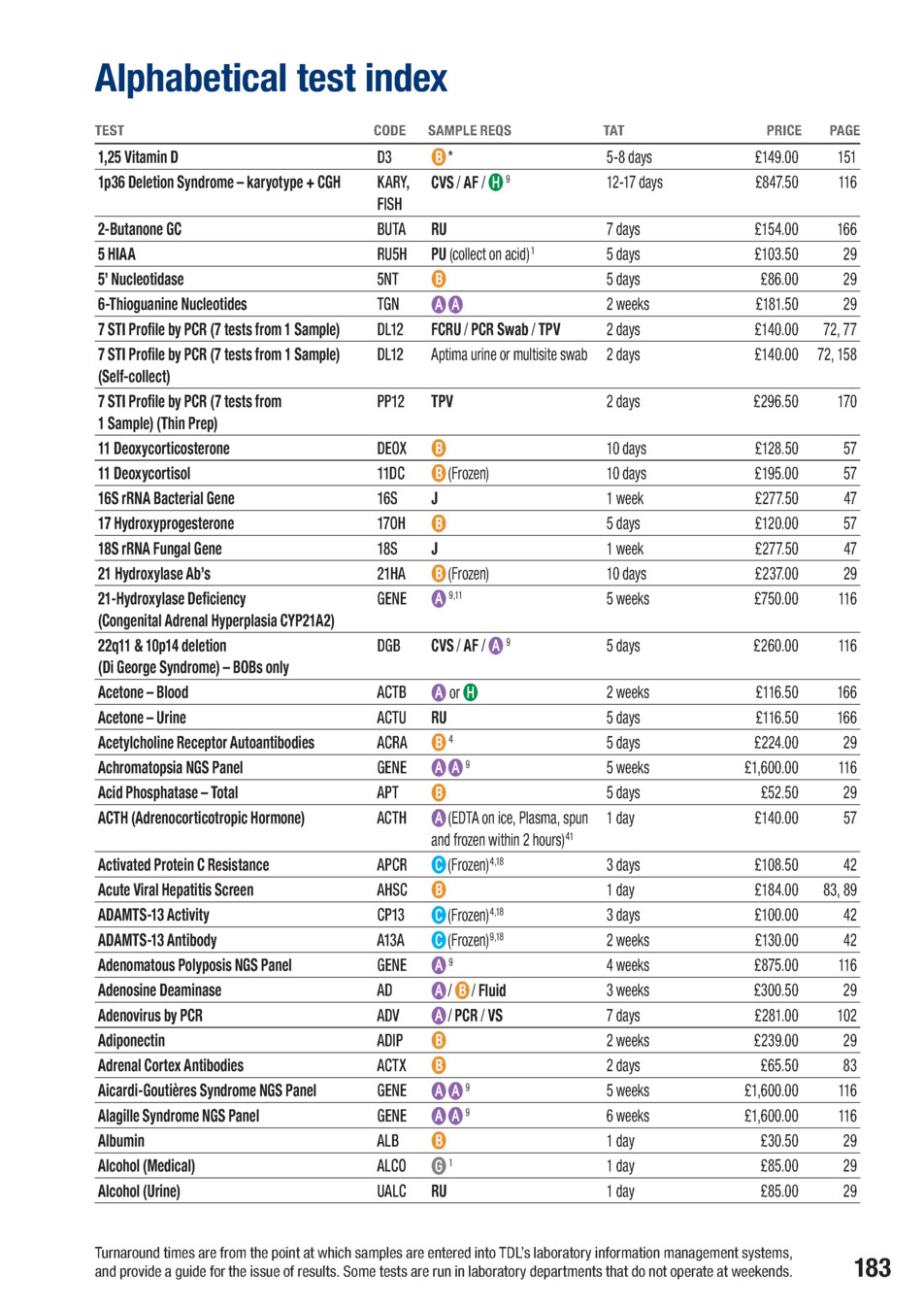
The document presented is an extensive Alphabetical Test Index produced by a medical laboratory catalog, designed to inform clinicians, researchers, and patients about the wide array of tests available within a single reference framework. It organizes offerings by test code and category, providing essential details for each entry, including the required specimen type or collection method, turnaround time (TAT), and price. The index serves as a practical guide for planning diagnostic workups, pre-analytical requirements, and budgeting, while also highlighting the breadth of capabilities—from targeted biochemical assays to comprehensive genetic and genomic panels. It also notes that some tests are run in specific laboratory departments and that weekend operations may affect results. (Page: not specified)
A notable feature of the index is the inclusion of a diverse set of test modalities. The catalog spans metabolic and endocrine assessments, immunology and autoimmune panels, infectious disease serologies and PCR-based tests, as well as extensive genetic testing, including single-gene analyses, targeted next-generation sequencing (NGS) panels, and whole-panel workups. The mix reflects contemporary clinical practice where precise molecular characterization and biomarker profiling are increasingly integrated into routine diagnostics and research. (Page: not specified)
The structure of the listing typically presents a test code or description, the sample type(s) acceptable for the assay (for example, EDTA plasma, serum, whole blood, urine, CSF, or swabs), any special handling notes (such as freezing conditions or immediate processing requirements), the expected turnaround time, and the price. In several cases, self-collection options or alternative collection methods are offered, underscoring flexibility in specimen procurement. The catalog also draws attention to the importance of correct labeling and documentation, including patient identifiers and collection timestamps, as a prerequisite for accurate processing and reporting. (Page: not specified)
The price points in the index illustrate a broad spectrum, ranging from modestly priced biochemical tests to high-cost, multi-variant genetic panels. This variation mirrors the complexity of the analyses—where assays may involve extensive sequencing, multiple enzymatic or immunological targets, or integration of data across multiple platforms. The pricing framework provides a snapshot of the market landscape for these laboratory services and may be used to compare cost implications across different diagnostic strategies. (Page: not specified)
Categories and representative entries
Within the Alphabetical Test Index, several thematic groups emerge that capture the core areas of laboratory diagnostics. One prominent cluster centers on genetic testing, including targeted gene sequencing for conditions such as congenital syndromes, metabolic disorders, and endocrine defects. Users can expect entries describing gene panels with or without deletions/duplications, the testing modality (NGS, karyotype, CGH, PCR-based assays), and notes about specimen requirements. The presence of diverse genetic tests reflects the move toward precision medicine, where identifying specific pathogenic variants enables tailored clinical decision-making. (Page: not specified)
Immunology and autoimmune testing form another major cohort, featuring profiles that assess immune-related antibodies, autoimmune markers, and autoimmune disease risk. These panels often include both broad screening tests and targeted antibody panels, with attention to sample type (plasma, serum, CSF) and appropriate handling. The catalog demonstrates a commitment to capturing complex serology across organ systems, providing clinicians with a pathway to confirmatory testing and disease characterization. (Page: not specified)
Biochemical and metabolic assessments also populate the index, covering enzymes, metabolites, proteins, and classic chemistry markers. These entries emphasize the importance of precise specimen collection (for instance, urine or blood notations), timing considerations, and sometimes fasting status, given the influence of physiological states on test results. The range underscores routine clinical chemistry as a foundation for diagnosing a wide spectrum of conditions from organ function to nutritional status. (Page: not specified)
The infectious diseases domain is well represented, including serology panels, antigen tests, and nucleic acid amplification methods. The catalog highlights how microbiology results can be augmented by molecular techniques to enhance sensitivity and specificity, with explicit guidance on sample types and collection methods essential for valid results. This emphasis on multiple modalities supports accurate detection and characterization of infectious agents across diverse clinical scenarios. (Page: not specified)
Turnaround time (TAT) is a central thread throughout the index, with values ranging from same-day or next-day turnarounds for certain assays to several weeks for complex genetic panels or comprehensive profiles. The variability in TATs reflects the inherent complexity of each test, the volume of analyses, and the operational workflows of the laboratory. Clinicians are advised to consult the specific TAT for each test during ordering to set appropriate expectations for results and subsequent clinical decision-making. (Page: not specified)
The pricing landscape is diverse, with some tests priced modestly while others command higher prices due to sequencing depth, probe panels, or the inclusion of confirmatory steps. For readers planning test strategies, the price data provide a practical lens to weigh diagnostic yield against cost, particularly when choosing between single-analyte tests and multi-analyte panels. The catalog thereby supports cost-conscious decision-making in addition to clinical rationale. (Page: not specified)
The index includes frequent reminders about handling and documentation, such as ensuring specimens and accompanying forms are labeled with patient identifiers (forename, surname, date of birth) and precise collection details (date and time). These procedural notes reinforce the pre-analytical rigor required to maintain sample integrity and accurate linkage between specimens and reports, which is essential for reliable results in any laboratory setting. (Page: not specified)
In summary, the Alphabetical Test Index captures a panoramic suite of modern diagnostic offerings—from single-analyte tests to expansive genomic panels—alongside practical considerations for collection, processing, and interpretation. Its design supports clinicians in selecting appropriate tests, estimating turnaround times and costs, and coordinating complex diagnostic workflows that integrate genetic, metabolic, immunological, and infectious disease data. As laboratory science continues to advance, such catalogs serve as valuable navigational tools to align patient care with the most informative and timely tests available. (Page: not specified)
Practical takeaways for practitioners and patients: first, recognize the breadth of testing options beyond traditional single-analyte assays; second, leverage the self-collection and flexible sampling options when appropriate to improve accessibility and convenience; third, plan for variable turnaround times based on test complexity and department capacity, particularly for weekend or holiday constraints; and fourth, use the pricing cues to balance diagnostic yield with budget considerations while discussing options with the laboratory team. These insights can help optimize diagnostic pathways, avoid unnecessary repeats, and ensure that results are delivered in a clinically meaningful timeframe. (Page: not specified)
The document concludes with a nod to the broader publishing ecosystem surrounding clinical laboratory services, including digital flipbook presentations and resource sections that connect readers to galleries, catalogs, and reports. The closing notes acknowledge the year of publication and the platform hosting the material, situating the index within a modern, accessible framework designed to support ongoing education and informed decision-making in laboratory medicine. (Page: not specified)